
Uka-Okali Angela Juliet1, Ozims Stanley James1, Eberendu I.F1*, Uka Kalu Okali2 and Kalu Joy3
1Department of Public Health, Faculty of Health Sciences, Imo state University.
2Federal Medical Center Umuahia.
3Department of Public Health, Madonna University Elele.
*Corresponding Author: Eberendu I.F, Department of Public Health, Faculty of Health Sciences, Imo state University.
Received Date: December 19, 2023
Accepted Date: January 04, 2024
Published Date: March 23, 2024
Citation: Uka-Okali Angela Juliet, Ozims Stanley James, Eberendu I.F, Uka Kalu Okali and Kalu Joy. (2024) “Evaluation of Bacterial Contamination of Fruits and Vegetables in the Three Senatorial Zones of Abia State.”, International Journal of Medical Case Reports and Medical Research, 2(3); DOI: 10.61148/2994-6905/IJMCRMR/019.
Copyright: © 2024. Eberendu I.F. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The study assessed the level of bacterial contamination in fruits and vegetables across Abia State's three senatorial zones. That was being studied experimentally. Based on the morphology, structure, and features of the colony, the bacterial content of 360 sample sizes of fruits and vegetables were determined. These samples were gathered from various marketplaces within the three state zones. Five bacterial isolates with differing frequencies of occurrence were found in the results: Escherichia coli, Staphylococcus aureus, Salmonella sp, Klebsiella pneumoniae and Shigella sp. Amongst the bacterial isolates, Escherichia coli and staphylococcus aureus were isolated in samples from all the six markets and three zones. Escherichia coli: Abia South (42%), Central (35.5%) and North (23.5%). Other bacteria species were isolated from all the zones but not in all markets. Salmonella sp, Klebsiella pneumoniae and Shigella sp, were detected in (26,%), (12%), (18%), and (10%). The prevalence of salmonella sp isolate over klebsiella pneumoniae and shigella sp isolates was significant. The three senatorial zones of Abia State have statistically significant levels of bacterial contamination of fruits and vegetables, which can lead to common diseases. For this reason, government and public health officials need to implement programmes to raise awareness about the risks of eating contaminated food and how to reduce bacterial contamination of food.
Introduction:
Consuming unprocessed or processed fruits and vegetables from markets may pose a risk to public health due to bacterial contamination. Fruits and vegetables' microbic flora are influenced by things like human handling, waste materials, and manure that introduce non-resident microorganisms [1]. Consuming fruits and vegetables from open markets has been linked to a number of ailments [2]. During food-related outbreaks, pathogens such as Enterobacteria and Escherichia coli have been linked to increased illness prevalence [3].For instance, in Nigeria, correct handling of commodities, market sanitation, and the personal hygiene of fruit and vegetable vendors are occasionally overlooked, resulting in the contamination of fruits and vegetables. To maximise the nutritious benefits, fruits and vegetables must be handled carefully to prevent infection. Fruits and vegetables must be thoroughly cleaned to prevent contamination. It has been observed that adding different amounts of organic acids to water, such as sorbic, carboxylic, and acid, will lessen the effect of bacteria on fruits [4]. Naturally occurring, non-pathogenic bacteria from their environment and decay are present in fruits. Nevertheless, handling and packing procedures could contaminate them [5]. This is a result of inadequate internal control and fruit and vegetable processing gone wrong.
The majority of foodborne illness cases resulting from bacterial contamination of food go unreported. Among the most prevalent foodborne diseases are Bacillus cereus, Escherichia coli, Salmonella typhi, species of Shigella and Vibrio cholerae, which cause diarrhoea, typhoid fever, dysentery and cholera, gardiasis, strongyloidiasis, and taeniasis etc [6]. Fruits and vegetables tainted by the aforementioned pathogens may result in serious foodborne illnesses and even death, depending on a number of variables including the type of microorganism and therefore the socioeconomic status and health of the consumers. These microbes' toxins are a primary cause of fruit and vegetable contamination. Continuous microbiological monitoring is required because consuming food infected with microorganisms poses a serious risk to public health and food safety.
Materials and Methods:
Collection of Samples:
A total of 180 fruits and 180 vegetable samples (5 different types of fruits and vegetables from each market) were collected in sterile bags from 6 open markets (2 markets from each senatorial zone). The markets are; Eke-Amiyi and Eke Elu (Abia North), Ubani Central market and Orie Ntigha (Abia Central), Ahiaohuru and Ariaria (Abia North). The fruit samples were Guava (Psidium guajava), Orange (Citrus sinensis), Lemon (Citrus limon), Apple (Malus domestica) and Mango (Mangifera indica) while the vegetable samples were Waterleaf (Talinium triangulare), Fluted pumpkin (Telfairia occidentalis), Carrot (Daucus carota), Cabbage (Brassica oleracea) and Eggplant (Solanum melongena). The samples were immediately taken to the microbiology laboratory for analysis.
Microbial Analysis:
Preparation of Materials:
The media were sterilized to avoid contamination from media: glassware, petri-dishes, test tubes, pipettes, flasks and bottles were all sterilized in a hot oven at 1700C for two hours and distilled water sterilized by autoclaving for 15mins at 1210C.
Isolation and Identification:
Ten (10) grams of the selected raw vegetables and fruits each were measured and finely chopped aseptically. The weighed samples were washed in 100ml of sterile distilled water. 1ml of the wash water was then subjected to ten-fold serial dilution series. 1ml from 104 dilutions were spread plated in duplicate onto Nutrient Agar plates and MacConkey Agar plates and then incubated at 37oC for 24 hours [7]. Identification of bacteria was done using colony characteristics and biochemical properties of the isolates. Pure cultures were sub-cultured onto nutrient agar slants and stored at 4oC in a refrigerator and the following done:
Gram’s Staining:
This was used to distinguish Gram negative and Gram positive organisms. In this form of staining, a thin smear was made on a clean grease- free glass slide. The smear was air dried and heat fixed by rapidly passing the smear over the flame of Bunsen burner three times. The fixed smear was flooded with crystal violet for 60 second. After 60 seconds, the stain was rapidly washed off with distilled water and then flooded with Lugol’s iodine solution for one minute. The iodine was washed off with distilled water and the smear was decolorized with acetone for 15 seconds. The slide was washed immediately with distilled water and counterstained with safranin for 60 seconds. After that, the stain was washed off with distilled water and air dried. The smear was then observed under the microscope using oil immersion with X100 objective lens.
Catalase Test
2ml of hydrogen peroxide solution was poured into a test tube and a glass rod was used to remove some colonies of the test organism and immersed in the hydrogen peroxide solution. Observation for Bubbles of oxygen gas 5- 10 seconds after was done immediately.
Oxidase Test:
For presence of the enzyme cytochrome oxidase in the test organism, a piece of filter paper was placed in a clean Petri dish and three drops of freshly prepared Kovac’s oxidase reagent was added. A glass rod was used to remove a colony of the test organism and smeared on the filter paper and then allowed for 10 seconds. Development of purple colour would indicate a positive result while no purple colour formation would indicate a negative result.
Indole Test:
The test organism was inoculated into a test tube containing 3ml of sterile tryptophan broth and incubated at 37oC 24 hours. 0.5ml of Kovac’s reagent was then added. The mixture was shaken gently and examined for red colour on the surface layer within 10 minutes. The production of red layer would indicate a positive result.
Citrate utilization Test:
This biochemical test was done to identify those organisms which have the ability to use citrate as the only carbon source. For this test, Simon’s citrate agar was prepared, dispensed in test tubes, autoclaved and solidified in a slanting position. Bacterial isolates were streaked from fresh cultures and the tubes were incubated at 37oC. Results were recorded after 4 to 7 days of incubation, respectively.
Sugar Fermentation:
This test was carried out to determine the ability of the test organism to metabolize sugar with the production of acid or gas. The following sugars were prepared and used for the test-glucose, maltose, lactose, arabinose, fructose, sucrose, mannose and mannitol. In the test, 0.2g of each of the sugars was dissolved in 20 ml of peptone water solution. Phenol red was added as indicator and 5 ml aliquots were dispensed into test tubes containing Durham tubes and autoclaved at 121oC for 15 minutes (121oC for 3 minutes for lactose, sucrose, arabinose and maltose as they may break down if sterilized further). It was then allowed to cool and then inoculated under aseptic conditions with the test organism using sterile wire loop and afterwards incubated at 37oC for 24 hours. A change in colour from purple to yellow indicates a positive result while gas production was shown by the presence of bubbles in the Durham tubes.
Methyl Red Test:
The medium for this test is glucose phosphate broth also called Methyl Red-Voges-Proskauer broth. It was prepared by mixing 5g of peptone and 5g of dihydrogen phosphate in one litre of distilled water. The mixture was steamed until the solid dissolved after which it was filtered. Five grams of glucose was added and mixed well. It was then distributed in 5 ml volume into test tubes and sterilized at 121oC for 15 minutes. During sterilization, the tubes were placed on a solid bottom container to protect them from contact with steam as this may make the medium become straw yellow in colour. After sterilization, the medium was allowed to cool and the test organism was inoculated into the broth and incubated for four to seven days at 37oC. After incubation, 5 drops of the indicator methyl red were added to the broth. It was then observed for the formation of red colour after few minutes to indicate a positive result while yellow colour was taken as negative result.
Voges-Proskauer Test:
The medium for this test is also glucose phosphate broth (Methyl Red-Voges-Proskauer broth). The medium was prepared, sterilized and inoculated as stated in Methyl Red test. After incubation for 24 hours, 0.6 ml 0f 5% α-naphtol and 0.2 ml of 40% aqueous Potassium hydroxide (KOH) were added and the test tube shaken. The result was recorded after10 to 15minutes and the formation of a red ring would indicate a positive result.
Urease Test:
An overnight culture of the test organism was streaked on the surface of urea agar slant under aseptic conditions. The cap of the test tube was closed loosely and incubated at 37°C for 48 hours for up to 7 days. It then examined for the development of a pink colour after 4 to 7 days of incubation.
Coagulase Test:
In this test, a drop of water was placed on each end of a slide. A colony of the test organism was emulsified in each of the drops to make thick suspension. A loopful of plasma was added to the suspension and mixed gently. The clumping of the organism was checked within 10 seconds.
Results and Discussion:
Identification of Bacterial Isolates:
Morphological and physiological characteristics of the bacterial isolates were investigated according to the method described by Bergey et al, 2000.
|
|
|
|
BIOCHEMICAL TEST |
|
|
SUGAR FERMENTATION |
|
|||||||||
|
S/N |
CULTURAL MORPHOLOGY |
Microscopic Features |
Catalase |
Coagulase |
Indole |
Methyl -red |
VP |
Urease |
H2S |
Citrate |
Oxidase |
Lactose |
Glucose |
Motility |
Gram Reaction |
ISOLATES |
|
1 |
Pink colonies on Macconkey Agar |
Rod Shape |
+ + |
- |
+ - |
+ - |
- + |
- + |
- |
- + |
- - |
+ + |
+ + |
+ - |
- |
Escherichia coli |
|
2 |
Mucoid Pink colonies on Macconkey agar |
Rod Shape |
+ |
- |
- |
- |
+ |
+ |
- |
+ |
- |
+ |
+ |
- |
- |
Klebsiella Pneumoniae |
|
3 |
Creamy colonies with rough edges on Nutrient agar |
Cocci in clusters |
+ |
+ |
- |
+ |
+ |
+ |
- |
- |
+ |
+ |
+ |
- |
+ |
Staphylococcus aureus |
|
4 |
Colourless colonies with black centres on SSA |
Rod Shape |
+ |
- |
- |
+ |
- |
- |
+ |
- |
- |
- |
+ |
+ |
- |
Salmonella sp |
|
5 |
Colourless colonies on SSA |
Rod Shape |
+ |
- |
+ |
+ |
- |
- |
- |
- |
- |
- |
+ |
- |
- |
Shigella sp |
Taxanomic identification using biochemical tests confirmed the isolates to be Escherichia coli, Staphylococcus aureus, Salmonella sp, Klebsiella pneumoniae and Shigella sp.
Table 1: Morphological and Biochemical Identification of Isolates
The total count of bacteria isolated from fruit and vegetables samples in all the six markets were recorded.
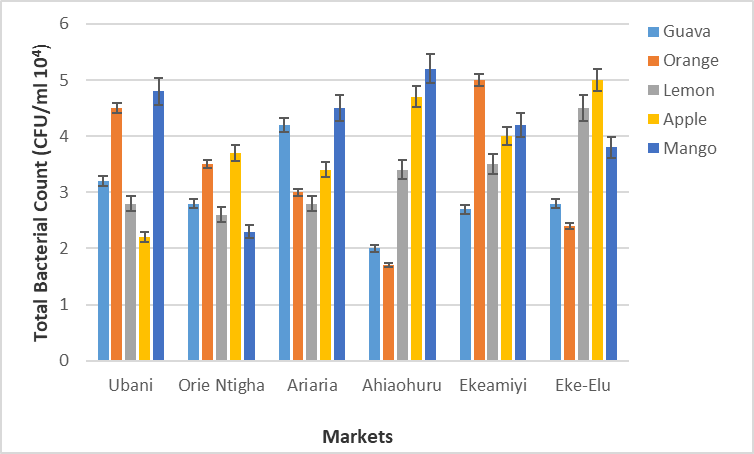
Figure 1: Total bacterial (viable plate counts) from fruit samples (CFU/ml X 104).

Figure 2: Total Bacterial (viable plate count) from Vegetable Samples (CFU/ml X 104).
Figure 2 Displays bacterial counts from vegetable samples in the six senatorial zones. Optimal bacterial counts from vegetable samples were observed in carrot purchased from Ahiaohuru market (4.2 X 104). Counts in Orie Ntigha market were also high with isolates from waterleaf samples having a count of 3.9 X 104. However waterleaf purchased from Ubani and Ariaria markets had lower counts (1.8 X 104 and 1.7 X 104 respectively). Lowest counts were observed in eggplant from Ekeamiyi and cabbage from Ubani markets (1.6 X 104).
Percentage occurrence of bacterial isolates in the three Senatorial Zones:
Figure 4. displays the percentage occurrence of bacteria isolates in Abia Central Senatorial zone. Escherichia coli was the highest occurring isolate with (34.4%) occurrence. This was followed by Staphylococcus aureus with (20.2%) occurrence. There was significant statistical difference in the occurrence rate of bacteria isolates in Abia Central zone, Salmonella Sp 18%, Klbesiella 16.5%, Shigella 12%.
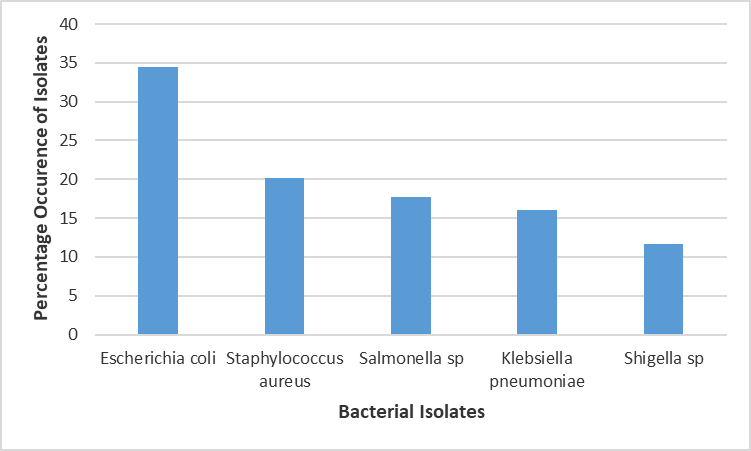
Figure 3: Percentage Occurrence of Bacterial Isolates in Fruit and Vegetable Samples in the Abia Central Senatorial Zone.
Figure 4 displays occurrence rate in percentage of bacteria isolated from fruits and vegetables in Abia South zone. Escherichia coli was the highest occurring with (42%) while Klebsiella pneumoniae had the least with (8%) occurrence. There was significant statistical difference in the occurrence rate of bacteria isolates in Abia South, Staphylococcus aurous 22%, Salmonella Sp 15%, Shigella 12.5%.
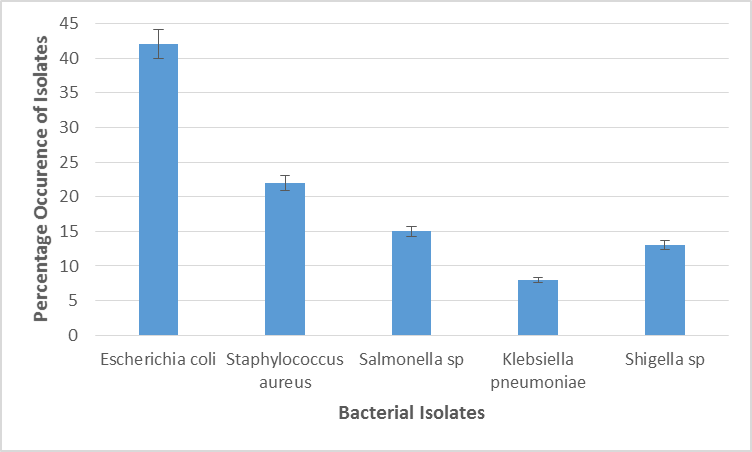
Figure 4: Percentage Occurrence of Bacterial Isolates in Fruit and Vegetable Samples in the Abia South Senatorial Zone.
Figure 5 exhibits occurrence rate of bacteria isolates in samples from Abia north zone. Escherichia coli had the highest occurrence (34%) followed by Staphylococcus aureus (26%), Salmonella sp (12%), Klebsiella pneumoniae (18%) and Shigella sp (10%). There was significant statistical difference in the percentage occurrence of bacteria species isolated from samples in Abia North.
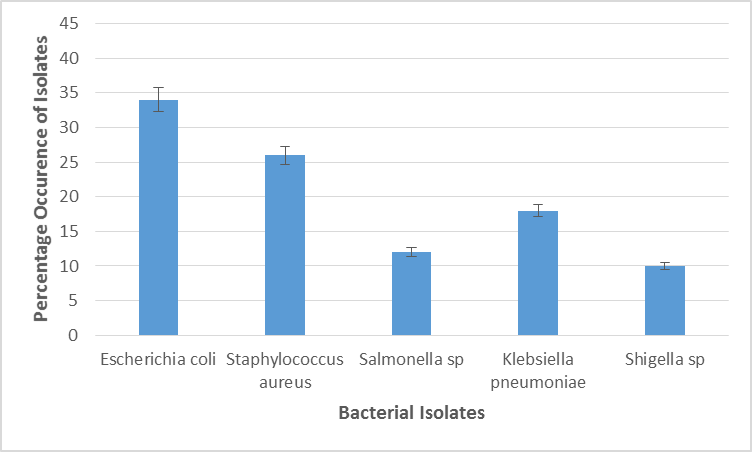
Figure 5: Percentage Occurrence of Bacterial Isolates in Fruit and Vegetable Samples in the Abia North Senatorial Zone
Comparative occurrence of bacterial isolates in the three Senatorial Zones:
The five different bacterial isolates exhibited varying levels of occurrence in samples analysed across the three zones. This is displayed in figure 5. Escherichia coli was the highest occurring bacteria species in all the three zones with the highest occurrence observed in Abia south (42%). The second most occurring isolate in the three zones was Staphylococcus aureus. Salmonella sp isolates were more prevalent than Klebsiella pneumoniae and Shigella sp in Abia Central but were less prevalent than Klebsiella pneumoniae in Abia North with 15% occurrence. Shigella sp were the least prevalent isolates in Abia central and Abia North but were more prevalent than Klebsiella pneumoniae in Abia south with 13% occurrence. Klebsiella pneumoniae isolates were the least prevalent in a zone with only 8% occurrence in Abia South.
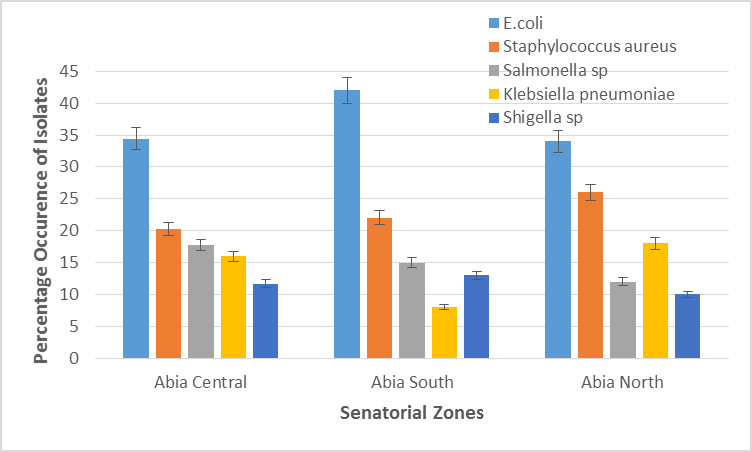
Figure 6: Comparative percentage occurrence of bacterial isolates in the three senatorial zone
Conclusion:
This investigation recorded a high load of bacteria isolates in fruits and vegetables sold in markets across the three senatorial zones of Abia state, Nigeria. Microorganisms identified are both of health importance and spoilage value. This analysis found that fruits and vegetables sold in marketplaces throughout the three senatorial zones of Nigeria's Abia state had a significant load of isolated bacteria. The found microorganisms have both nutritional value and are important for health. Because vitamins are necessary for a healthy lifestyle, fruits and vegetables must be safe for human consumption. One of the main causes of bacterial contamination of fresh produce in Abia state is poor sanitation in market areas. Fruits and vegetables are typically cleaned with tainted water before being shown on unclean tabletops and the ground. Additionally, the sellers' lacklustre maintenance of cleanliness is a factor. The continual infection of microorganisms is ensured by all these variables. Another issue that contributes is the usage of contaminated water for growing fruits and vegetables. To guarantee that the public is informed of the health hazards connected to consuming tainted fruits and vegetables, the state government should launch awareness campaigns.